mosquito Crystal for Protein Crystallisation Screening
The TTP Labtech mosquito Crystal is the protein crystallographer’s choice for liquid handling. It has been designed specifically for protein crystallisation screening to provide faster, more cost-effective and easier sample preparation.
Benefits For Protein Crystallisation Screen Preparation
TTP Labtechs mosquito Crystal liquid handler offers the following benefits for screen preparation:
- Use of smaller sample volumes which extends the reach of precious protein samples resulting in cost savings and more extensive screening
- Accurate reproducible pipetting from 25nl to 1200nL.
- Precise low volume liquid handling across a wide range of viscosities
- No risk of cross contamination through the use of disposable tips
- Rapid set up up time (<2min)
- Automation of popular protein crystallisation techniques and no need to change instrument settings e.g. hanging drop, sitting drop, microbatch, seeding or additive screening plate preparation with no need to change instrument settings
- High accuracy means drops can be places centrally in the sub-wells of sitting drop plates every time. This facilitates automated plate imaging, making protein crystals easier to identify
- The ability to create several multi-component drops per well in 96-well hanging drop set ups. This allows the simultaneous analysis of different protein concentrations, ligands or complexes
- mosquito’s unique nanolitre liquid handling technology can perform multiple aspirations before a single dispense. This is essential for auomated seeding and direct addition of additive screens to the drops.
- The ability to dispense a combination of solutions simultaneously, with the addition of mixing if required, providing perfect drop formation for optimal protein crystallisation
- No need to recalibrate between experiments
- Simple operation suits both first time users and seasoned operators
Specifications
| Dispense range | 25nL - 1200nL |
| Plate/Deck capacity | 2 or 5 |
| Experimental setup type | hanging drop, sitting drop, microbatch, bicelle |
| Plate set up time | <2 mins |
| Dead volume | <0.3 μL |
| Min accessible volume | 10nL |
| Dimensions (w x d x h) | 390 x 470 x 690 mm |
| Weight | 27kg |
| Services | 220V single phase 50/60 Hz |
| Noise | 64 dBA peak noise during operation |
| Optional extras | Humidity chamber |
Development of New Flu Drugs Using Protein Crystallography
Pandemic and world-wide emergence of Tamiflu-resistant seasonal human influenza has highlighted the need for the ongoing development of new anti-virals, efficient production of vaccine proteins and novel diagnostic tools. Analysis of drug-protein interactions using X-ray crystallography can be used to elucidate the key molecular contacts which lead to inhibition. These interactions can be optimised to establish strong anti-viral drugs. However this pioneering work requires large quantities of protein. Prof. Kurt Krause, Otago University, New Zealand and his group have been establishing a novel eukaryotic expression system for influenza neuraminidase (NA). Ashley Campbell, an MSc student has worked on expressing an NA protein in this system that is suitable for X-ray crystallographic studies.
Miniaturising the Crystallisation Process
The influenza surface glycoprotein NA is essential for the efficient spread of the virus. When inhibited, viral spread between hosts is limited and symptoms of the disease decrease rapidly. Miniaturising the optimal expression system for an NA protein has proved to be challenging. The initial stages required Ashley Campbell to set up small-scale crystal screens in order to find suitable conditions for crystallisation. Choosing these conditions can be a daunting experience when you need to conserve limited pure protein sample and set up very small and accurate drop sizes.
Using the mosquito® Crystal robot, crystallisation drop sizes were reduced to 150 nL, allowing more conditions to be screened. Ashley set up crystal drops using the hanging drop method on 96 well plates, containing various crystallisation conditions. The multi-aspirate function of mosquito Crystal allowed for the aspiration and mixing of 150 nL of protein sample with 150 nL of each crystallisation condition. Crystal hits occurred in 27 out of the 192 conditions (Fig 1a) allowing many opportunities to optimise the crystal (Fig 1b) from only a small protein sample.
Further X-ray diffraction of these crystals produced crystals into high 2Å ranges (Fig 2).
accuracy and precision Reducing dispensing volumes to nanolitre amounts requires accuracy and precision that is not possible using manual methods. Automating this process enables consistency in pipetting and minimise operator error. The volume range capabilities of liquid handling systems vary considerably and are dependent on on the type of dispensing technology. The accuracy of mosquito Crystal is unrivalled mainly due to its unique positive displacement pipetting. Prof. Krause commented, The mosquito Crystal has made it possible to work with more proteins that are only available in small amounts. This means that new projects can be started more readily and new vista are opened up for scientific inquiry.
It also boasts a system of disposable micropipette tips that reduce the likelihood of cross contamination, remove the need for time-consuming wash steps or replacing costly, fixed-tip dispenser heads. The pipette tip design also allows the tip to reach the bottom of a well leaving minimal (less than 0.3 ?L) dead volume in the source well and no in-tip dead volume. This allows you to keep all the savings from an efficient set-up process.
From a students point of view Ashely Campbell commented, I have found mosquito Crystal very user-friendly which has meant that I can work independently and still produce high quality crystals.
The mosquito Crystal has made it possible to work with more proteins that are only available in small amounts. This means that new projects can be started more readily and new vista are opened up for scientific inquiry
 Ashley Campbell is an MSc student in biochemistry, at the University of Otago. She works with Prof. Kurt Krause who is Director of Webster Centre for Infectious Diseases at the university. Ashleys research focuses on the expression and purification of the influenza neuraminidase protein in a eukaryotic cell line for the identification and characterisation of novel inhibitory drugs.
Ashley Campbell is an MSc student in biochemistry, at the University of Otago. She works with Prof. Kurt Krause who is Director of Webster Centre for Infectious Diseases at the university. Ashleys research focuses on the expression and purification of the influenza neuraminidase protein in a eukaryotic cell line for the identification and characterisation of novel inhibitory drugs.
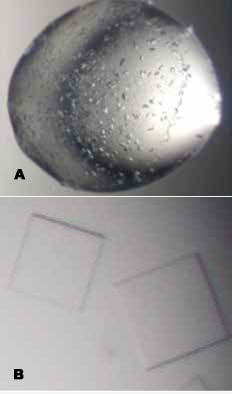
Figure 1. Crystals of influenza neuroaminidase.
(a) A 300 nL drop, set up by mosquito Crystal in a crystal screen. The crystallisation condition containing 12% PEG-20,000, 0.1M MES, pH6.5 was further optimised to produce thin plate crystals (b).
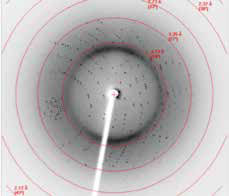
Figure 2. Good diffraction pattern of an optimised influenza neuraminidase crystal showing clean isotropic diffraction and well resolved spots into the high 2 Å ranges (in house data).


 Download the AXT Life Science Product Guide
Download the AXT Life Science Product Guide



