Q-Phase Multimodal Holographic Microscope for Live-Cell Imaging
A unique holographic microscope for label-free live-cell automated cytometry
The Q-Phase is a multimodal holographic microscope is a unique instrument that expands the possibilities of light microscopes in the areas of biology and biotechnology. It performs Quantitative Phase Imaging (QPI) based on the patented technology called Coherence-controlled holographic microscopy and has been specifically designed to observe living cells in vitro.
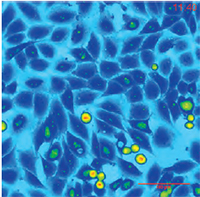 | 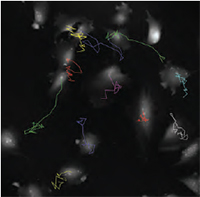 | 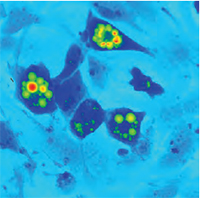 |
| QPI of non-metastatc fibroblast-like LW13K2 (K2) cells. | QPI of rat sarcoma cells, cell tracking. | QPI of human adipocytes. |
System Design
The system itself is based around an inverted transmission optical microscope and uses incoherent light sources (halogen lamp and LED). It incorporates an incubator to ensure specimen integrity is maintained.
With electric motors driving all of the components, automation makes even the most complex and time consuming operations a pleasure. To aid user-friendliness and to assist those already familiar with other TESCAN instruments, their characteristic control panel with touchscreen control can also be used. The high level of automation allows the Q-Phase to perform multidimensional acquisition achieving timelapse, channel, position and Z-stack imaging.
Imaging Modes
- Quantitative phase image (QPI) can provide information on sample morphology, topography or cell dry-mass distribution
- It provides very simple and sensitive way for monitoring of cell reactions to treatment and analyses of movement, growth or various other parameters (area, shape, )
- Thanks to high-quality imaging with Q-PHASE, cell boundaries can be very precisely detected
The Q-Phase can also be optioned with the following multiple imaging modes:
- Widefield fluorescence imaging (attachment module)
- Simulated DIC imaging
- Brightfield imaging
- Reflection holographic microscopy
- Side port available for other techniques
Applications
The Q-Phase is an indispensable research tool for applications such as:
- Testing the response of cells to particular treatments even with scattering non-transparent substances
- Monitoring the life cycle of cells during mitosis
- Distinguishing between different forms of cell death
- Analysis of cell growth
- Looking at motility or morphology changes
- Imaging cells in extracellular matrices
Key Features
Key features of the Q-Phase multimodal holographic microscope include:
- No image artifacts such as halo effect (as opposed to techniques based on Zernike phase contrast illumination)
- Enables very precise detection of cell boundaries
- Strong suppression of coherent noise (speckles) & parasitic interferences (as opposed to laser-based approaches)
- Label-free no staining is needed, simple sample preparation, observation of live cells in their native environment, no photobleaching problems
- Low phototoxicity low light power density (107× lower than fluorescence microscopy) allows long-term observations (for days)
- Coherence-gating effect Q-PHASE special feature enabling to observe samples even in scattering media (phospholipid emulsions, extracellular matrices, etc.)
- Multimodality fully integrated fluorescence module, simulated DIC and brightfield which enables automatic multimodal imaging of the sample
- High-quality QPI unique Q-PHASEs optical setup allows using incoherent illumination which provides extraordinary imaging quality without any compromises
- Lateral resolution of conventional microscopes (up to 2× better when compared to common laser-based approaches or pinhole spatial filtering based techniques)
- Fast acquisition the use of off-axis holographic approach makes Q-PHASE a single-shot instrument, thus enabling imaging of very fast cell dynamics
- Full motorization focusing, sample stage, objective exchange, fluorescence filters
- Automated multidimensional acquisition time-lapse, channel, position, Z-stack
- Simple image segmentation and processing comparable to fluorescence data processing
- Quantitative phase values can be recalculated e.g. to cell dry-mass density (pg/?m2) or direct topography with nanometer sensitivity (usually non-biological samples with homogeneous refractive index distribution)
- High phase detection sensitivity enables to detect even the smallest changes in axial direction, very sensitive detection of morphology or position changes
Applications
Many different types of samples can be observed including adherent cells in monolayers, thin tissue sections or plant samples.
Sample preparation is straightforward simply requiring the operator to pour the cell suspension into the observing chamber with no further procedures needed. The cells can be also seeded into the perfusion chamber and perfusion system and different treatments can be applied. Cell reactions to the treatment can then be observed online.
Application examples
- ?Cell life cycle, cell proliferation, cell differentiation, cell viability, counting ?
- Cell dry mass evaluation, cell growth, changes in cell dry mass distributions
- Cell morphology changes, intracellular processes
- Cell motility, tracking
- ?Cell interactions, cell co-cultures ?
- Fast cellular processes
- Testing reactions of cells to a pecific treatment, cytotoxicity
- Imaging in scattering non-transparent media (e.g. phospholipid emul-sions), imaging in 3D environments (e.g. collagen extracellular matrix)
- Multimodal imaging (QPI automatically correlated with fluorescence, DIC or brightfield)
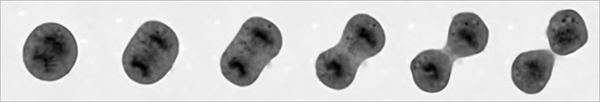 |
| QPI of rat sarcoma cell mitosis (inverted LUT, contrast enhanced by adaptive contrast control method) |
 |  |
| QPI of human adipocytes | QPI of human breast cancer cells |
 | 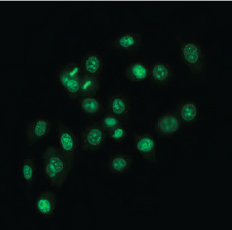 |
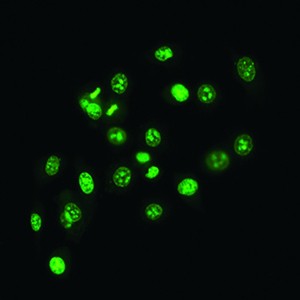
| QPI of olive leaf trichomes | Fluorescence image of human prostate cancer cells (SYTO 16) |
Intrinsic Imaging Modes
Complementary image contrast can be obtained just by numerical processing of the acquired phase images. In this way simulated DIC images can be produced with adjustable shear and displayed in real time.
Another possibility is the brightfield imaging which can be simply achieved by closing the reference arm of the microscope.
In summary, the Q-PHASE offers multiple imaging modes widely used in biological research such as fluorescence or DIC integrated in a single instrument and supported by the Q-PHASEs software allowing fully automated multimodal imaging.
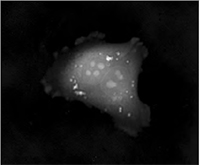 | 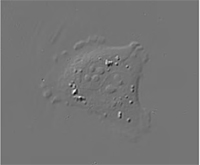 | 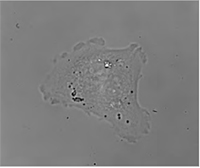 |
| Quantitative Phase Imaging (QPI) | Simulated DIC | Brightfield |
Fluorescence Imaging
The Q-PHASE can combine holographic microscopy with the fluorescence microscopy. This powerful combination provides the possibility to verify structures or processes observed in QPI with fluorescence microscopy in the same field of view using a single instrument. For example, morphological and position changes prior to cell death can be observed in QPI with following fluorescence verification of cell death types (see images below).
This approach greatly reduces the phototoxicity and photobleaching problems of fluorescence imaging and it allows long-term observations. The focus plane in both methods is located at the same position. This allows easy and fast switching between the two imaging methods at the same conditions and time points.
Multiple fluorescence channels are possible with motorized channel exchange for automated multidimensional measurements. The illumination can be implemented by using liquid light guide coupled solid state light sources or a xenon arc lamp. Multidimensional image acquisition combining holography and fluorescence is fully integrated in the Q-PHASEs software. A fluorescence module is attached to the side port of the Q-PHASE, which can alternatively be used for other imaging techniques.
Multimodal Imaging of Human Prostate Cancer Cells
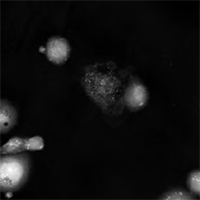 | 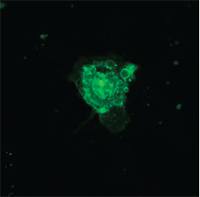 | 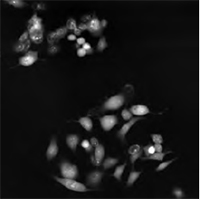 | 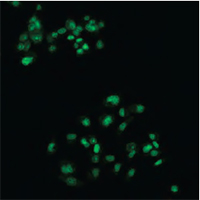 |
| Quantitative Phase Image | Fluorescence image (Annexin V) | Quantitative Phase Image | Fluorescence image (SYTO 16) |
Imaging in Scattering Media
A special feature of Q-PHASE is the coherence-gating, a well-known effect in optical coherence tomography which enables observations of samples even in scattering media. This effect is induced by using incoherent light in the unique patented setup of Q-PHASE. Its transmitted-light configuration enables to effectively suppress the light which was scattered by the environment in defocused planes and to only use unscattered light for imaging. In this way, cells can be observed even in moderately scattering non- trans-parent substances such as an active phospholipid emulsion.
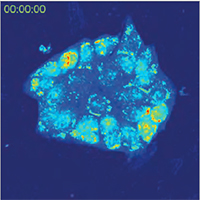 | 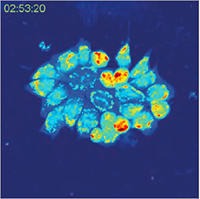 | 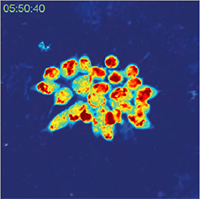 |
| QPI of reaction of human colorectal cancer cells to treatment by 0.15% active phospholipid emulsion (scattering medium). | ||
Imaging in Extracellular Matrices
The coherence-gating effect can also be beneficial when imaging cells in extracellular matrices such as collagen gel. Extracellular matrices mimic in vivo environments making the study of the cells dynamic reactions to its surroundings more realistic. Usually it is used as a biological test for cancer cell invasivity and ability to metastasis.
The Q-PHASE microscope enables to record mechanism of cell motion and interactions between extracellular matrix fibers and cells with high contrast and without any additional staining.
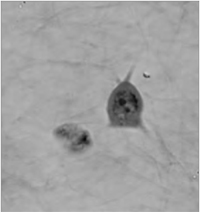 | 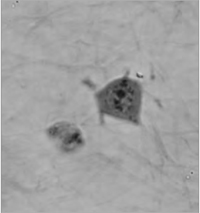 | 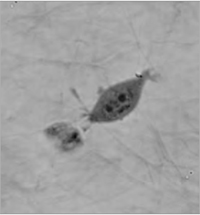 |
| QPI of human sarcoma cell motion mechanism in collagen gel (inverted LUT). | ||
Introduction to the Q-Phase Multimodal Holographic Microscope
Cell Mitosis
Automated Time-Lapse Segmentation Using the TESCAN Q-Phase
QPI Time Lapse of Cell Interactions – Entosis of Cancer Cells
Cells in Collagen Gel Viewed Using the Q-Phase Multimodal Holographic Microscope
Recording of the a human sarcoma cell and its reaction to collagen fibres in a simulated extracellular matrix. The video shows how the cell produces prolonged protrusions which appear to attach to the collagen fibrils which it uses to assist with a degree of mobility. It has the advantage over Zernicke-based methodologies in that it does not exhibit a halo artefact. Furthermore, the low coherence of the illumination is not impaired by the presence of the matrix.
Human Colorectal Cancer Cells Viewed Using the Q-Phase Multimodal Holographic Microscope
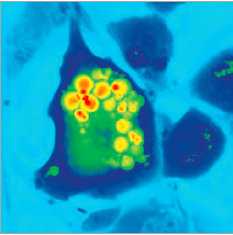
This recording is of human colorectal cancer cells and their reaction to treatment with 0.15% active phospholipid emulsion for nearly 6 hours. While the active phospholipid emulsion behaves as a scattering medium, the Q-Phase still generates clear images of the reaction of the cancer cells. Red indicates areas of higher cell mass density and blue-green areas lower cell mass density.
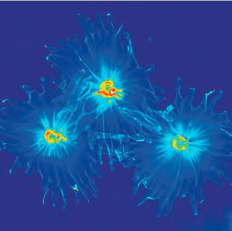
Proliferation or Rat Sarcoma Cells
This video shows rat sarcoma cells proliferating over 14 hours clearly showing cell division. The non-invasiveness and label-free nature of the Q-Phase make it ideal for this type of application.
Cell Morphology Analysis Using the TESCAN Q-Phase
Q-Phase software used to evaluate the principal characteristics of cell morphology and cell mass. The first part of the video shows quantitative phase imaging of plumbagin-treated PC-3 cell deaths on the left and segmentation of cells on the right part of the Q-PHASE control software window. Later on, the analysis of morphological changes in evaluated parameters, such as cell area, cell perimeter, circularity and cell mass are shown. Different types of cell deaths can be detected and distinguished according to the analysis.